SAR: Safety 1
Ryaltris was well tolerated with similar incidences of AEs compared with placebo or individual monotherapies.
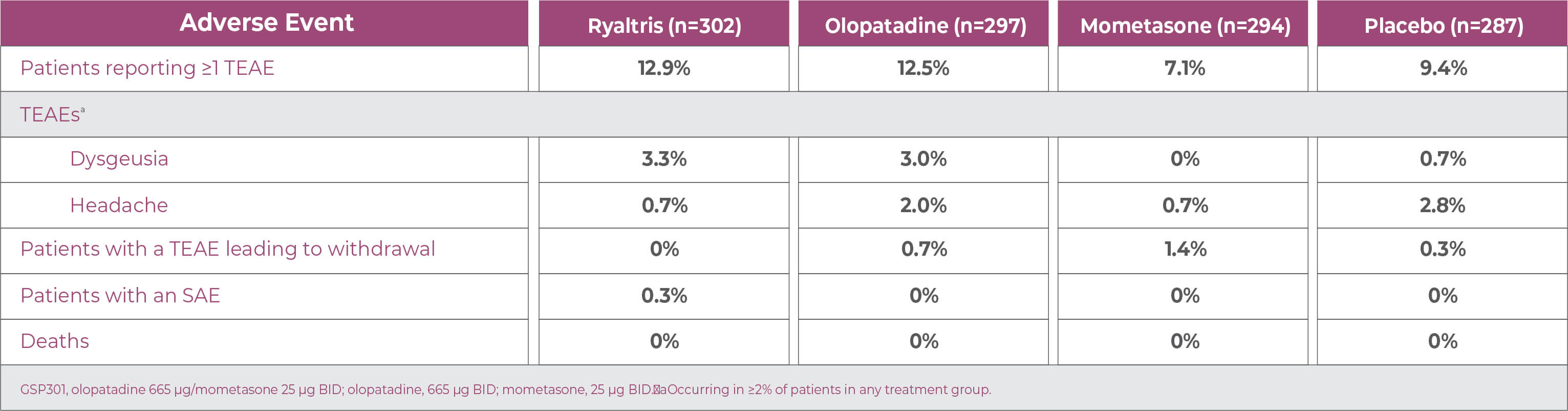
GSP301 is a combination of olopatadine hydrochloride and mometasone furoate. AE, adverse event; BID, twice daily; SAE, serious adverse event; SAR, seasonal allergic rhinitis; TEAE, treatment-emergent adverse event. 1. Hampel FC, et al. Allergy Asthma Proc. 2019;40:261-272; data on file.
PAR Study: Safety Results 1
At week 52, the incidence of adverse events was comparable across treatments. Most adverse events were mild to
moderate in severity
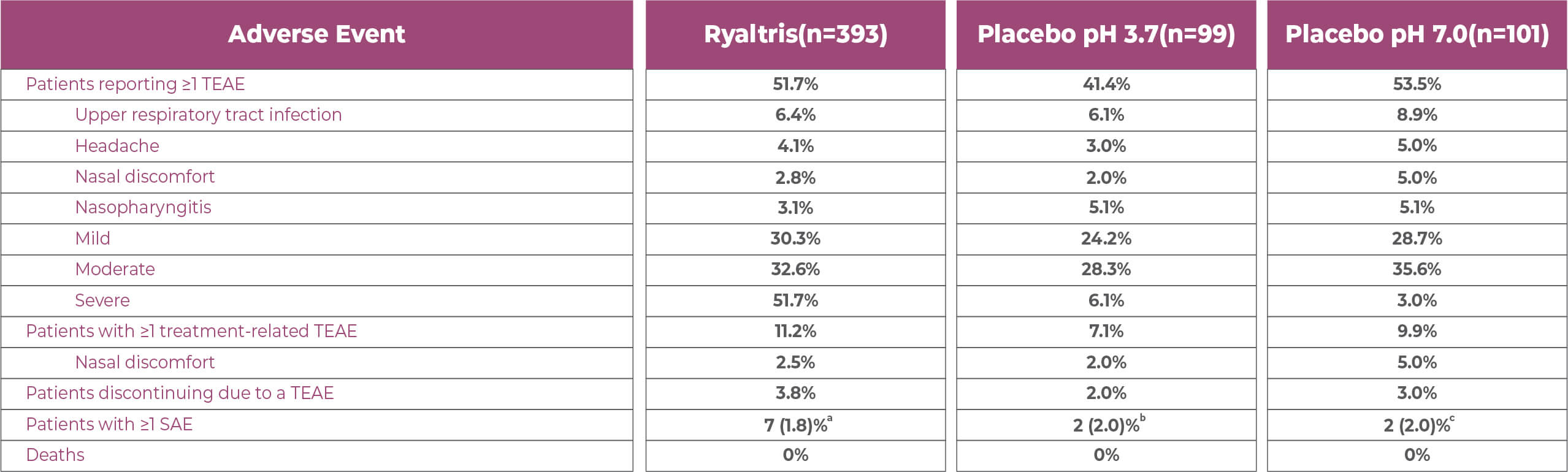
a1 pneumonia; 1 cellulitis; 1 cholelithiasis; 1 renal cell carcinoma; 1 anaplastic astrocytoma; 1 invasive ductal breast carcinoma. b1 appendicitis; 1 ectopic pregnancy. c1 nephrolithiasis; 1 cholelithiasis. GSP301 is a combination of olopatadine hydrochloride and mometasone furoate. PAR, perennial allergic rhinitis; SAE, serious adverse event; TEAE, treatment-emergent adverse event. 1. Segall, et al. Allergy Asthma Proc. 2019;40:301-310; data on file.