Nasal Symptoms 1
Efficacy and Safety of Olopatadine/Mometasone Nasal Spray in Treating Seasonal Allergic Rhinitis in Pediatric Patients
Significant improvements in all individual nasal symptoms with GSP301 vs placebo.
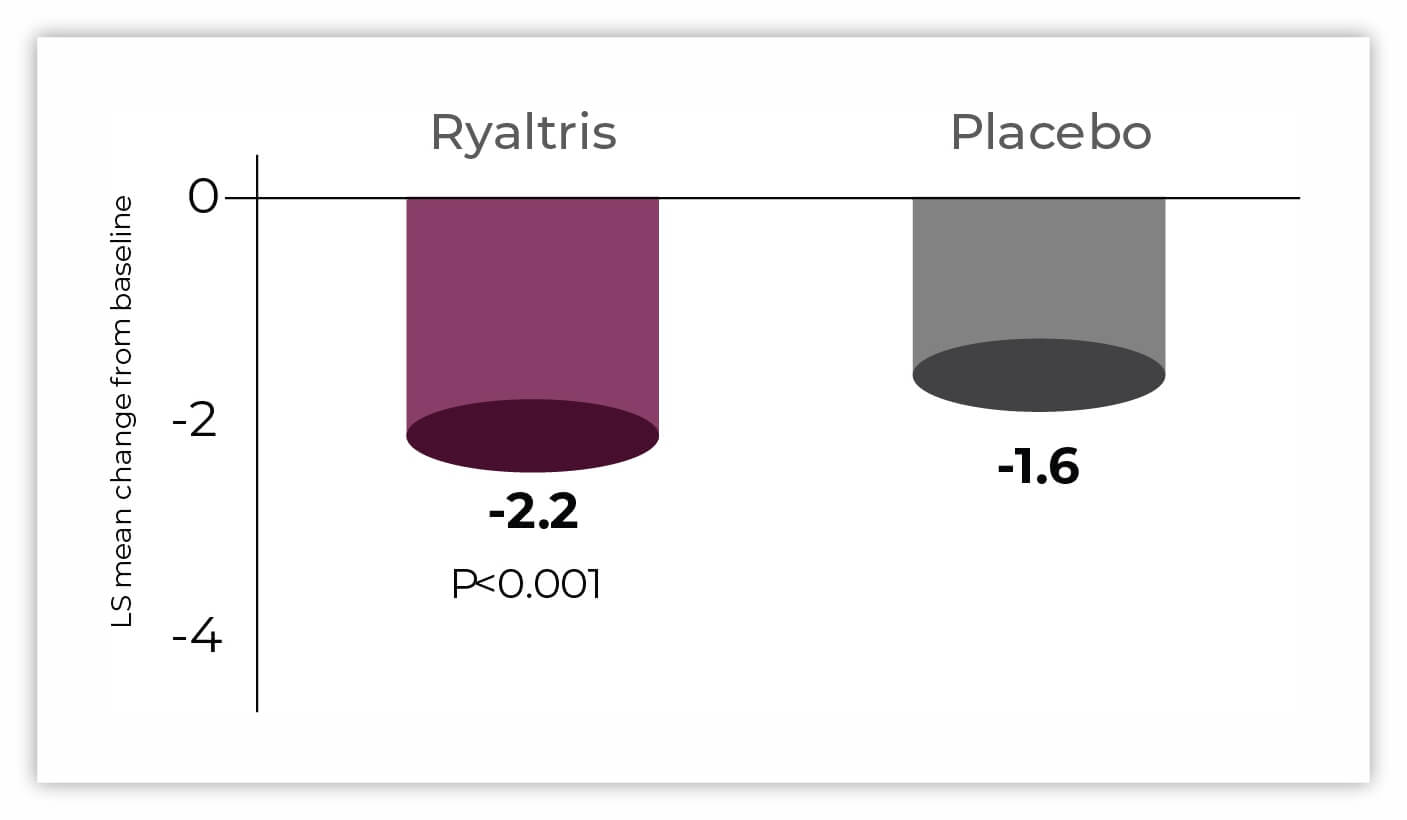
Significant improvements in all individual nasal symptoms with Ryaltris vs placebo (secondary efficacy).
1. Gross, et al. Ann Allergy Asthma Immunol. 2019;122(6):630-638; data on file. GSP301 is a combination of olopatadine hydrochloride and mometasone furoate. LS, least squares; rTNSS, reflective Total Nasal Symptom Score; SAR, seasonal allergic rhinitis. Data on file – GSP301-305 Clinical Study Report.
Ryaltris provides improved nasal symptom relief vs monocomponents
Significant and clinically meaningful improvements in rTNSS.
Significant improvements in all individual nasal symptoms with Ryaltris vs placebo (secondary efficacy).
• Ryaltris vs placebo (days 1-14)
• Olopatadine vs placebo (days 1-10)
• Mometasone vs placebo (days 1-14)
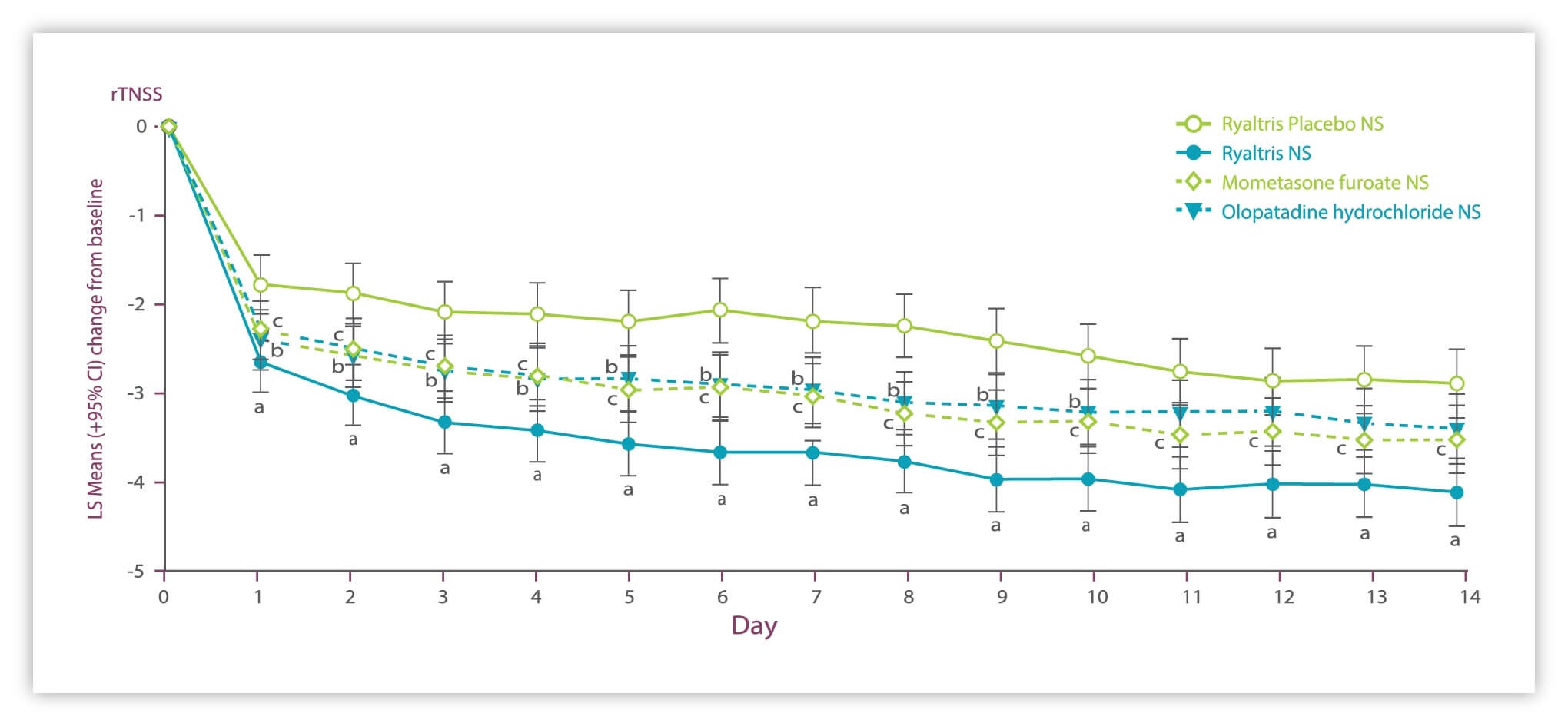
GSP301 is a combination of olopatadine hydrochloride and mometasone furoate. LS: Least squares NS: Nasal spray rTNSS: reflective Total Nasal Symptom Score SAR: seasonal allergic rhinitis. 1. Gross, et al. Ann Allergy Asthma Immunol. 2019;122(6):630-638; data on file.
Onset of Action1
A rapid onset of action (based on mean change from baseline in iTNSS from 15 minutes to 4 hours) was observed with Ryaltris vs placebo.
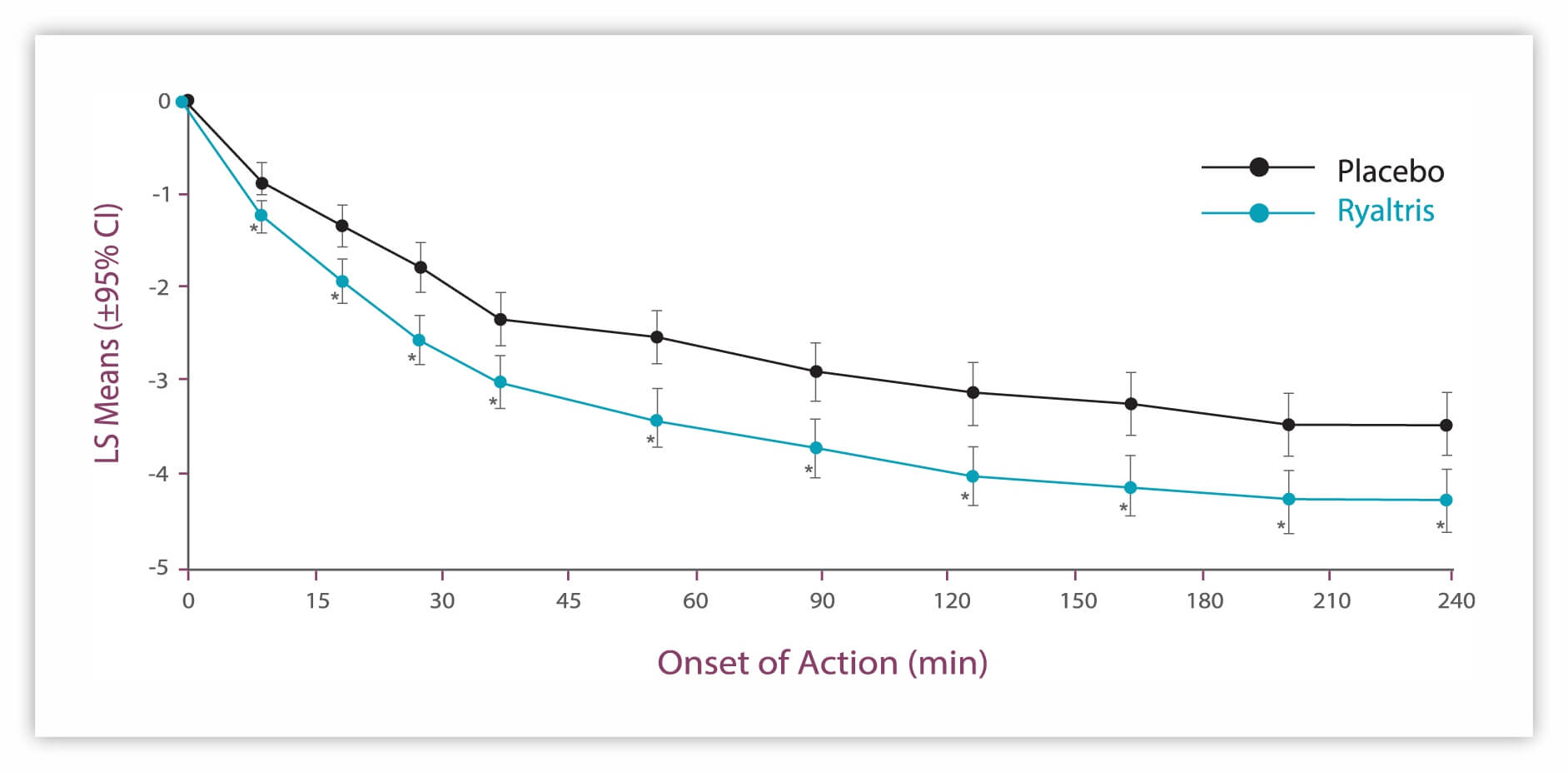
aSecondary efficacy GSP301 is a combination of olopatadine hydrochloride and mometasone furoate. CI: confidence interval iTNSS: instantaneous Total Nasal Symptom Score LS: least squares SAR: seasonal allergic rhinitis. 1. Hampel FC, et al. Allergy Asthma Proc. 2019;40:261-272; data on file.
Perennial Allergic Rhinitis
Clinically meaningful improvements in average 24-hour AM rTNSS and iTNSS over 52 weeks with Ryaltris vs placebo pH 3.7.
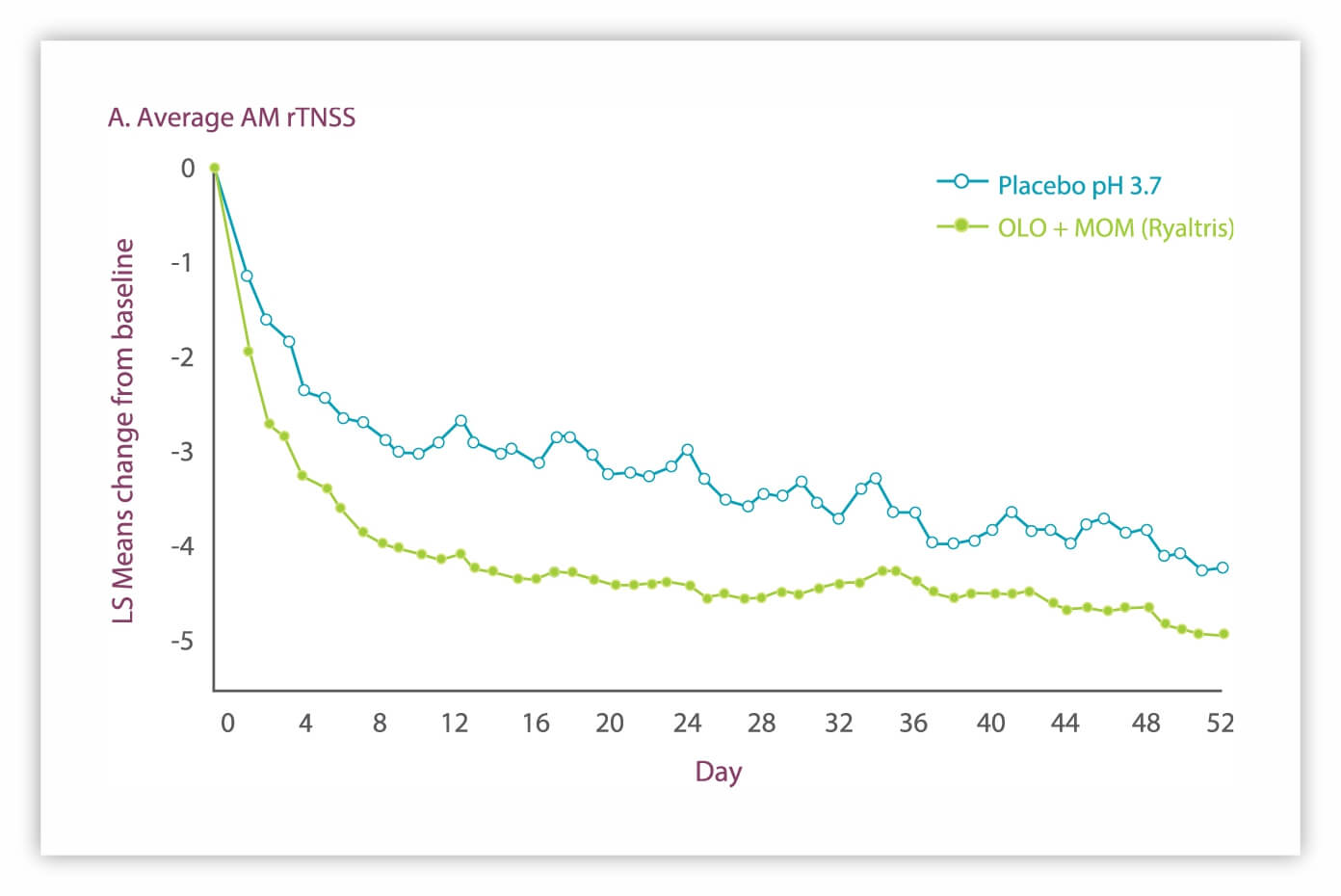
Clinically meaningful improvements in average 24-hour AM rTNSS and iTNSS over 52 weeks with Ryaltris vs placebo pH 3.7.

iTNSS: instantaneous Total Nasal Symptom Score LS: least squares PAR: perennial allergic rhinitis rTNSS: reflective Total Nasal Symptom Score aSecondary endpoint. GSP301 is a combination of olopatadine hydrochloride and mometasone furoate. 1. Segall, et al. Allergy Asthma Proc. 2019;40:301-310; data on file.
Ocular Symptoms 1
Significant and clinically meaningful improvements in rTOSS with Ryaltris vs placebo on each of the 14 study days.
Significant improvements in all individual ocular symptoms with Ryaltris vs placebo
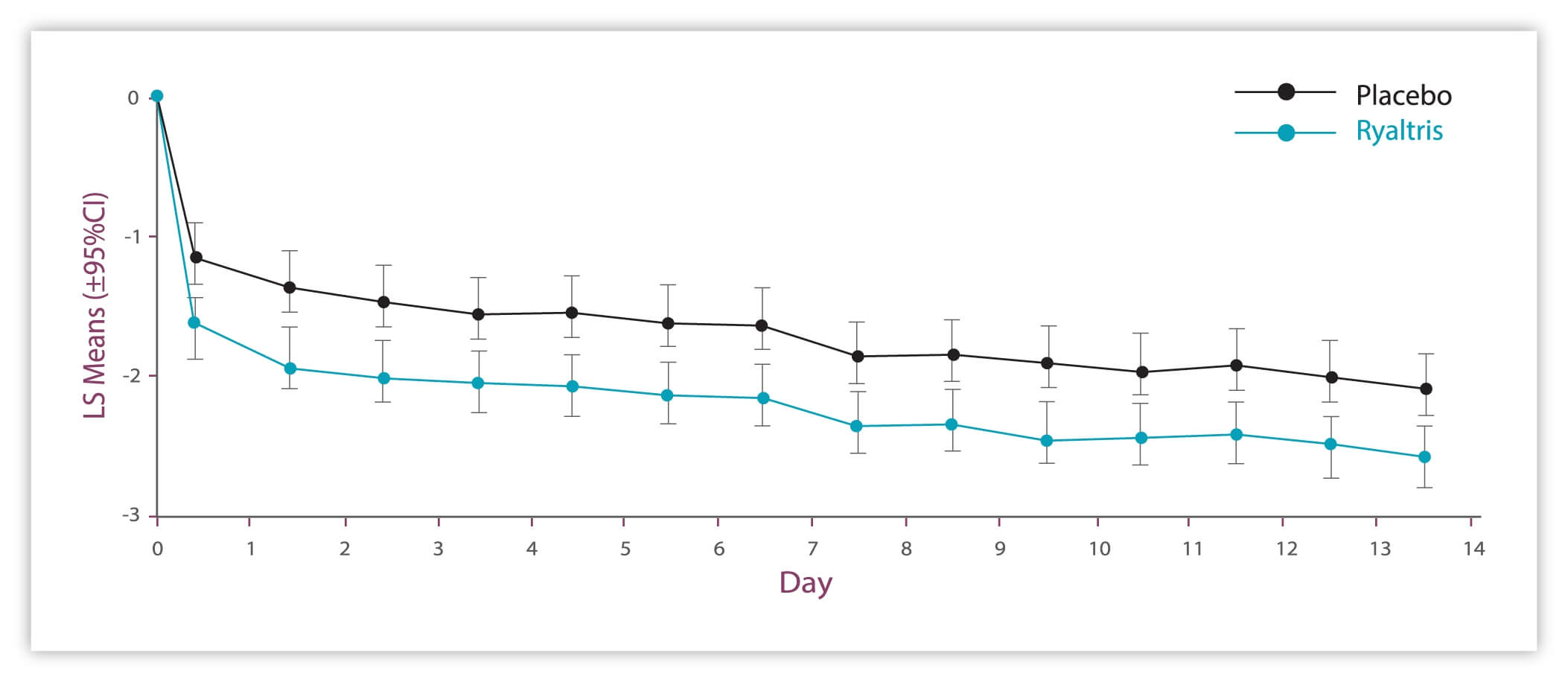
aSecondary efficacy. GSP301 is a combination of olopatadine hydrochloride and mometasone furoate. CI: confidence interval LS: least squares rTOSS: reflective Total Ocular Symptom Score SAR: seasonal allergic rhinitis. 1. Hampel FC, et al. Allergy Asthma Proc. 2019;40:261-272; data on file.
Quality of Life 1
Ryaltris significantly improved below mentioned seven individual domain scores compared with placebo:
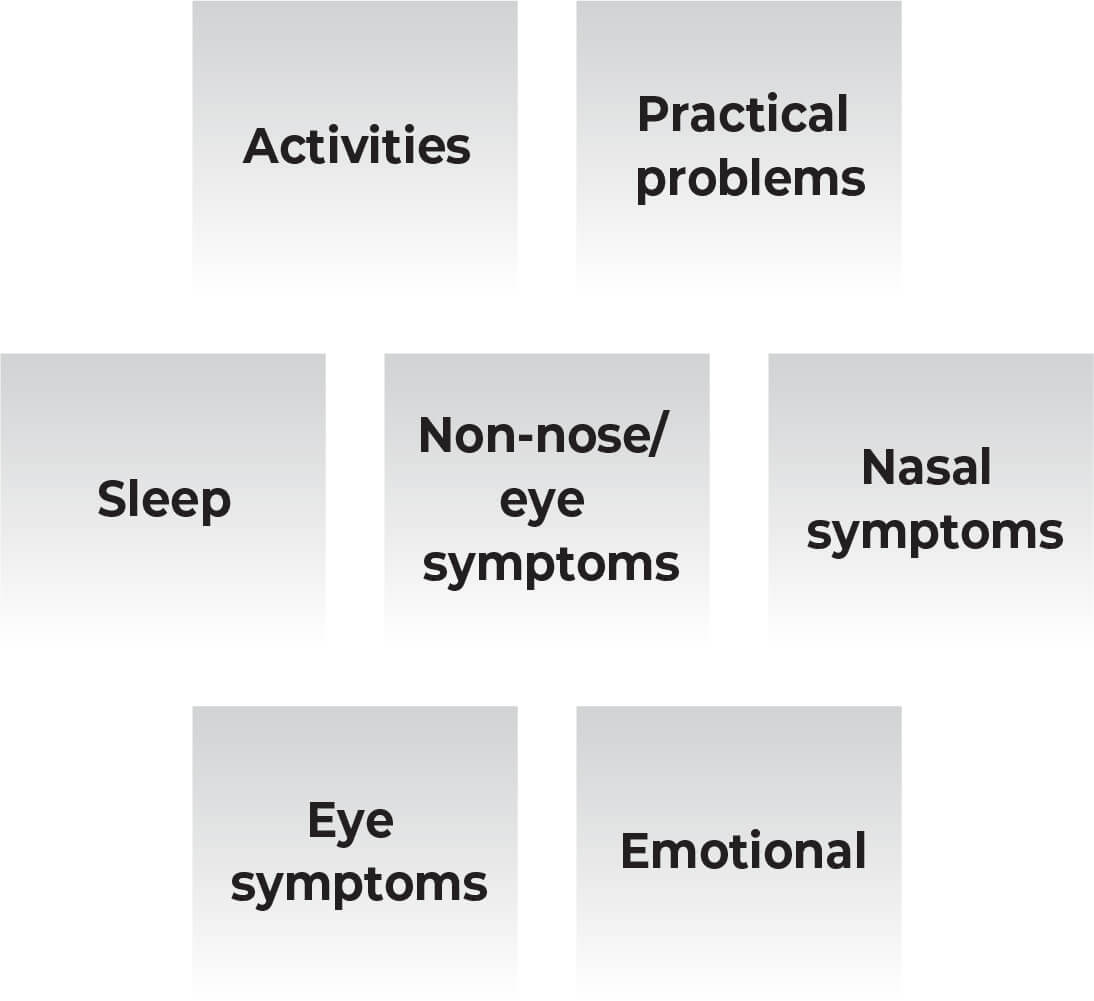
Ryaltris improved overall RQLQ scores vs placebo from baseline to day 15.
1. Adapted from Hampel et al.2019. MCID: minimum clinically important difference. RQLQ: rhinoconjunctivitis quality of life questionnaire.